Pregnancy is full of choices—but only a few come with a strict deadline. Umbilical cord cells can only be collected at birth, and once delivery is over, the opportunity is gone. If you want a clear plan (donation vs private banking), what to expect in the delivery room, and how to choose safely without falling for hype, book your consultation today.
What Umbilical Cord Cells Are
The phrase umbilical cord cells is often used as a broad, consumer-friendly term for valuable cells found in the umbilical cord and placenta after delivery—most commonly from cord blood and cord tissue. Cord blood can be donated to a public bank or stored privately for potential future use by the child or relatives.
You’ll also see this topic described as:
- umbilical cord blood cells (from cord blood)
- cord tissue cells (from cord tissue)
- Wharton’s jelly cells (a term commonly used when discussing the gelatinous tissue inside the cord)
- perinatal stem cells (a broader label for birth-associated sources)
Knowing which “bucket” you’re dealing with matters, because the evidence base, regulation, and typical use cases are not the same.
Cord Blood vs Cord Tissue: What’s the Real Difference?
Many families assume “cord banking” is one thing. In reality, it can mean two different collections.
Cord blood: the most established option
Cord blood contains blood-forming stem cells (hematopoietic stem cells). In the U.S., approved stem cell products are currently limited to blood-forming stem cells derived from umbilical cord blood for specific blood-related disorders—not for general wellness or “rejuvenation” uses.
What that means in practical terms:
- Cord blood has a clearer history of established clinical use (in specific medical contexts).
- It’s still important to avoid exaggerated claims about “treats everything.”
Cord tissue: a different biological resource
Cord tissue is often marketed because it can contain cell populations discussed in regenerative medicine.
Research reviews discuss the interest in umbilical cord/Wharton’s jelly–derived cells, including in orthopedic contexts, but this is not the same thing as universally proven, approved treatment for all conditions.
A safety-first rule:
If someone claims cord tissue products can treat multiple unrelated diseases as a routine service without clear oversight, be cautious—because regulators warn consumers about broad marketing of unapproved regenerative products, including those derived from Wharton’s jelly and other birth tissues.
Public Donation vs Private Banking
Your best choice depends on your goal.
Public donation (help others)
Public donation typically means your baby’s cord blood is stored for potential use by patients who need it, rather than reserved for your family. NHS Blood and Transplant describes donor eligibility screening and emphasizes suitability and safety checks for donations.
Why people choose donation:
- You want to help patients needing a match
- You’re comfortable that the unit won’t be reserved for your child
- Your hospital participates in a collection program
Private banking (reserve for your family)
Private banking is a paid service that stores the sample for potential family use. Cord blood contains hematopoietic stem cells with potential life-saving benefit and provides guidance for counseling patients about banking choices.
It licenses both public and private cord blood banks in the UK, reflecting the importance of regulated oversight.
What Happens During Collection
Most parents worry: “Will this disrupt my birth plan?” In properly run programs, collection is designed to happen after delivery and should not replace standard obstetric care.
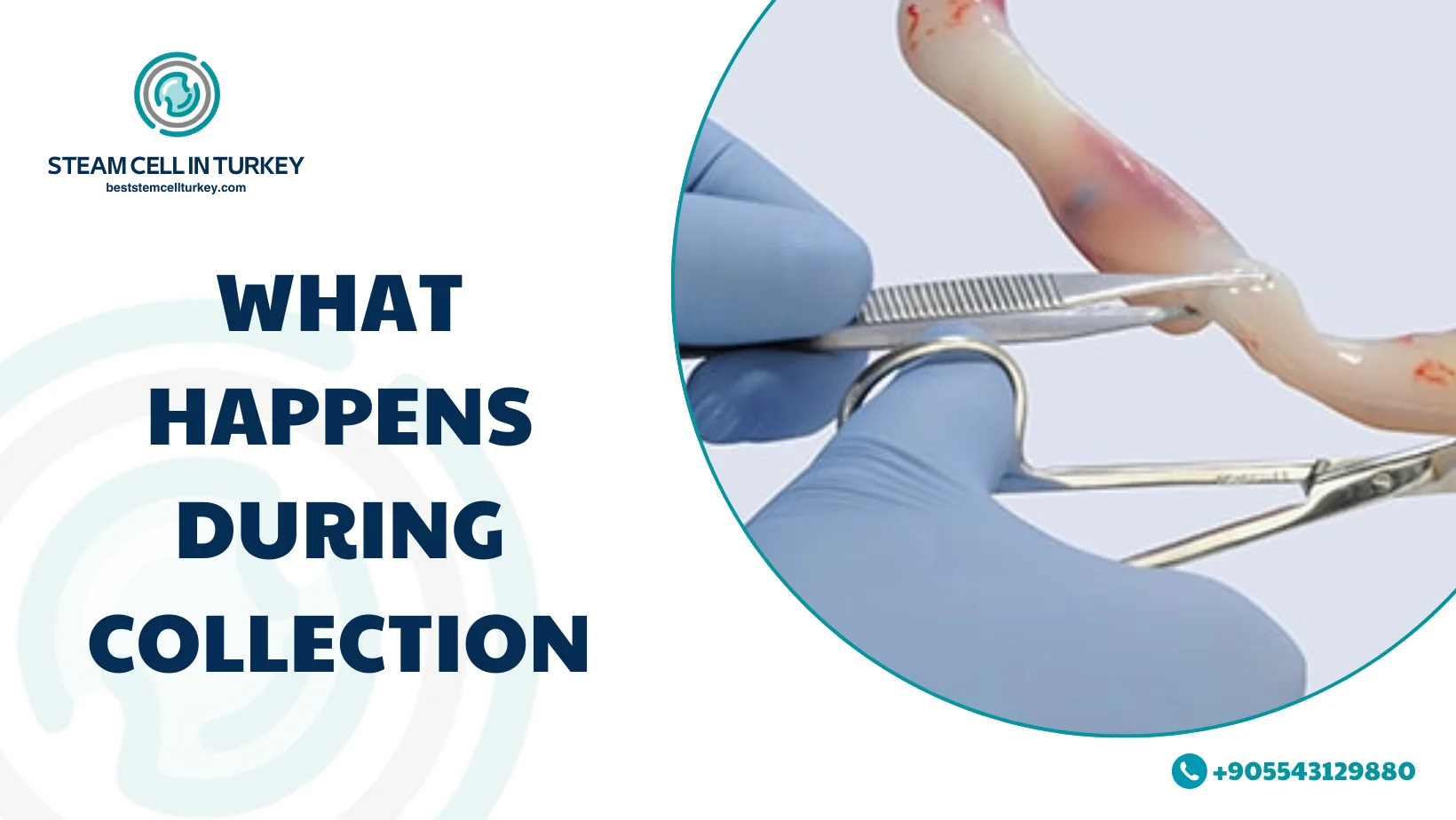
A simple real-world flow looks like this:
- You enroll before delivery and receive instructions (and often a kit)
- The delivery team confirms eligibility and timing
- Collection happens after birth from the cord/placenta
- The sample is labeled, transported, and processed for evaluation/storage
Donation is labeled with a unique number, recorded in the NHSBT database, then evaluated and processed in the cord blood bank laboratories if suitable.
Important note: not every collected unit is ultimately banked for clinical use—some are too small or don’t meet criteria. Canadian Blood Services, for example, lists reasons units may not be stored (like low volume/cell count or other operational/medical issues).
How to Choose a Safe Program (and Avoid Marketing Traps)
Because “stem cells” attract hype, your best protection is a quality checklist.
Look for:
- Transparent explanation of what’s being stored (cord blood, cord tissue, or both)
- Clear chain-of-custody and transport timing
- Documented infectious disease screening requirements (especially in donation pathways)
- Realistic language (no guaranteed cures)
- A clear explanation of what is approved vs what is experimental
The FDA warns consumers that there is broad marketing of unapproved regenerative medicine products and specifically mentions products derived from umbilical cord blood and Wharton’s jelly as examples in its consumer alert.
AABB also publishes patient-facing education that highlights what cord blood is, what it can do, and common misconceptions.
Costs: What You’re Really Paying For
Public donation is often free to the donor (depending on the country/program). Private banking costs vary based on:
- Cord blood only vs adding cord tissue
- Processing and testing fees
- Storage duration and annual fees vs long-term plans
- Courier and logistics
Before paying, ask for a written breakdown that separates:
- one-time processing/enrollment fees
- ongoing storage fees
- any future retrieval/activation fees
This protects you from “surprise” costs later and makes it easier to compare providers fairly.
Why Families Choose Our Team
At Best Stem Cell Turkey, we help families and patients make calm, informed decisions—without pressure. If you’re exploring umbilical cord cells options, we can explain:
- umbilical cord blood cells vs cord tissue cells in simple terms
- donation vs private banking and how to decide
- what safety documentation to request
- how to keep expectations realistic and evidence-aware
Visit our website and book your consultation here: Best Stem Cell Turkey
FAQs about Umbilical cord cells

Are umbilical cord cells the same as stem cell treatment?
No. Banking or donation preserves a resource; treatment depends on diagnosis, regulation, and evidence, and is a separate medical decision.
Can every cord blood collection be stored?
Not always. Programs may not store a unit if volume/cell count is too low or other criteria aren’t met.
What’s the safest way to compare banks?
Compare documentation, screening/testing standards, chain-of-custody, and clear explanations of what is approved vs experimental (not just price).
Are products from Wharton’s jelly cells automatically approved?
No. The FDA warns consumers about broad marketing of unapproved regenerative products, including those derived from Wharton’s jelly.
Is there a reliable patient guide for stem cell decisions?
Yes. The ISSCR treatment guide is designed to help patients understand how to evaluate stem cell interventions and avoid misleading claims.
The value of umbilical cord cells is timing: birth is the one moment you can collect and preserve this resource. If you want a clear, safe plan—whether donation or private banking—and guidance you can trust, book your consultation today.
References
- FDA — Cord Blood Banking: Information for Consumers FDA
- FDA — Consumer Alert on Regenerative Medicine Products (incl. Wharton’s jelly) FDA
- FDA — Important Patient & Consumer Information About Regenerative Medicine Therapies FDA
- ACOG — Umbilical Cord Blood Banking (Committee Opinion) ACOG
- AABB — Umbilical Cord Blood Donation FAQs AABB
- NHSBT (PDF) — Donating Cord Blood, Umbilical Cord and Placental Tissue NHSBT
- ISSCR — The ISSCR Guide to Stem Cell Treatments (Patient Handbook PDF) ISSCR
Ready to plan with confidence?
→ Book a Free Consultation
→ Explore Financing Options
→ contact with us via what’s up